Where to Find Cold Form Base Foil Manufacturers

The cold form base foil lies extremely high material requirements on the production capacity. Where to find a reliable cold form base foil manufacturer? Haomei Aluminum can provide it. Learn more.
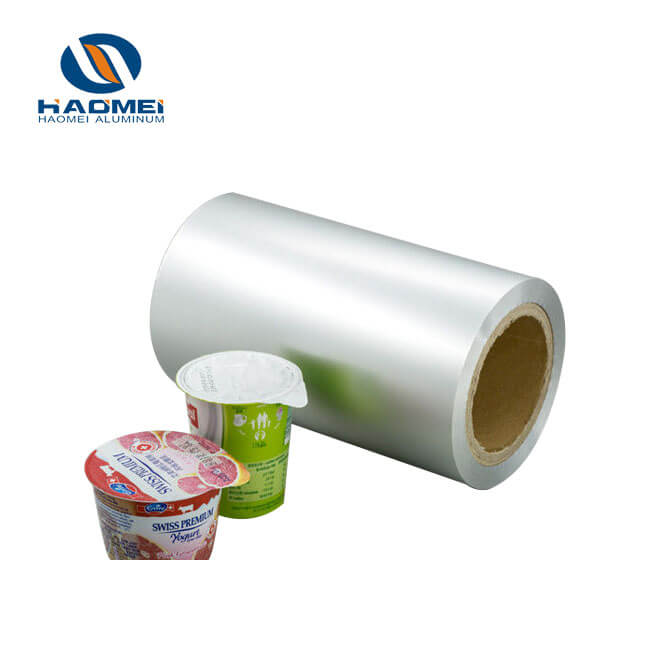
Cold-formed pharmaceutical foil, as a core material for high-end pharmaceutical packaging, has become the preferred packaging solution for vaccines, biological agents, and highly sensitive solid dosage forms due to its excellent barrier properties, formability, and safety.
However, what is little known is that behind this thin aluminum foil lie extremely high material requirements and production barriers, and finding reliable cold form base foil manufacturers is a major challenge for many pharmaceutical companies.

Cold-formed pharmaceutical foil's core substrate is aluminum foil, but it is by no means replaceable by ordinary aluminum foil. The special nature of pharmaceuticals requires its packaging to possess extreme barrier properties, mechanical strength, and cleanliness, which places almost stringent requirements on the material and performance of the aluminum foil.
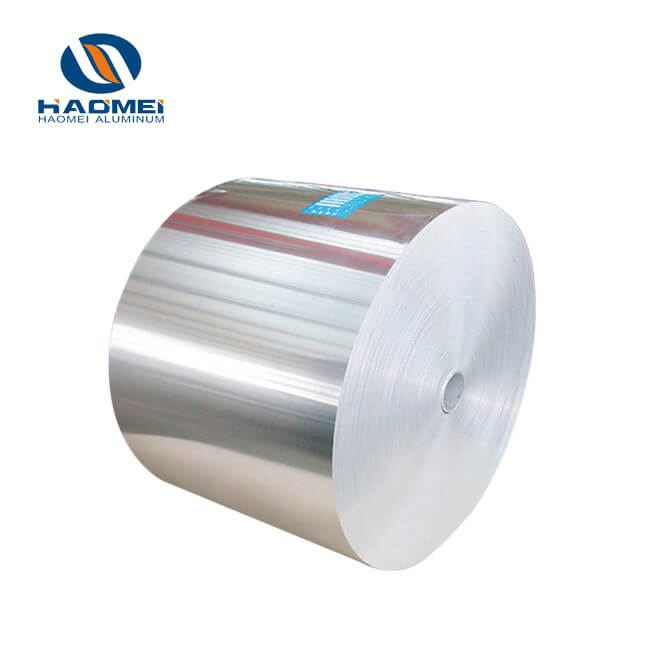
Firstly, in terms of material selection, cold-formed pharmaceutical foil mostly uses 8021-O-state aluminum alloy. This alloy requires precise control of the proportions of elements such as silicon, iron, and copper, with the iron content needing to be consistently between 1.25% and 1.65% to balance formability and strength. Any deviation in the element proportions can lead to cracking and damage during molding.
Secondly, precision and cleanliness control are crucial. The thickness of cold-formed pharmaceutical foil is typically between 0.04-0.065 mm, with a thickness tolerance controlled within ±2 μm; otherwise, the consistency of blister pack formation will be affected. Surface cleanliness is paramount; it must be free of defects such as black oil spots, scratches, and small black streaks, and the surface oil content must be below 13 mg/m² to prevent the drug from coming into contact with impurities and posing a safety risk.
Even more crucial is its barrier performance. The aluminum foil must be pinhole-free; industry standards require a pinhole density of ≤50 pins/m², while high-end products must control it to ≤20 pins/m² to effectively block oxygen, moisture, and light, extending the shelf life of the drug.
Production is challenging. Even with high-quality aluminum substrates, the production process of cold-formed pharmaceutical foil remains challenging. Every step requires precise control; even a slight mistake can lead to batch rejection. The production process involves multiple steps, including melting, casting, cold rolling, annealing, foil rolling, and slitting.
The annealing process alone requires multiple stages of temperature control—heating to 170-240℃ at a rate of 2.0-3.5℃/min and holding, then heating to 500-605℃ and holding for an extended period, finally slowly cooling to below 300℃. The entire process takes tens of hours, and a temperature deviation exceeding ±5℃ can affect the ductility of the aluminum foil.
The cold forming stage is even more critical. This process requires pressing the aluminum foil into a specific blister shape under low temperature and high pressure, demanding good cold-forming ductility. This necessitates eliminating internal stress through preceding cold rolling and annealing processes; otherwise, wrinkles and cracks are highly likely to occur during forming.
Furthermore, the entire production process must be carried out in a Class 10,000 or even Class 1,000 cleanroom, equipped with an online monitoring system and a full-process quality traceability system. An investment of over 300 million yuan is typically required for a cold-formed foil production line with an annual capacity of 10,000 tons. The high equipment and operating costs deter many small and medium-sized enterprises.
Haomei Aluminum
Due to stringent material requirements and high production barriers, high-quality cold-formed base foil suppliers are consistently in a state of "supply falling short of demand." Against this industry backdrop, manufacturers capable of consistently supplying high-quality cold-formed pharmaceutical foil with a full range of product capabilities are even rarer.
Leveraging years of technological accumulation in the aluminum foil processing field, Haomei Aluminum has precisely overcome the production challenges of cold forming foil. Its cold-formed pharmaceutical foil uses high-quality 8021-O temper aluminum alloy, strictly adheres to GMP standards, controls thickness tolerance within ±1.5μm, and has a pinhole density of less than 20 per m². All indicators exceed industry standards, perfectly meeting the packaging needs of high-end pharmaceuticals.
Furthermore, Haomei Aluminum has broken through the limitations of single-product supply, building a full-category pharmaceutical packaging material supply system. In addition to cold-formed pharmaceutical foil, it also provides PTP blister foil, tropical aluminum foil, and PVC and other plastic rigid sheets.
The PTP aluminum foil uses 8011 series aluminum alloy with a thickness of 0.016-0.04mm, suitable for conventional pharmaceutical packaging such as capsules and tablets; the tropical aluminum foil uses 8021 and 8079-O state aluminum alloys, providing a double protective barrier for high-end pharmaceuticals.
This "one-stop supply" model not only saves pharmaceutical companies time and costs in supplier selection and certification, but also ensures the compatibility and safety of different packaging materials through quality control throughout the entire supply chain, safeguarding the quality of pharmaceutical packaging from the source.
Inquiry
TABLE OF Contents

Haomei Aluminum CO., LTD.
Tel/Whatsapp: +86-15978414719
Email: sale@alumhm.com
Website: https://www.alumhm.com
Xin'an Industrial Assemble Region,Luoyang,Henan Province,China
Office Add: 1103, No.14 Waihuan Road, CBD, Zhengzhou, China